Ammoniak-Pufferlösung, Ammoniumchlorid/Ammoniak, pH-Wert 10, für die Chelatometrie, Honeywell Fluka™: Puffer Puffer und Lösungen | Fisher Scientific

Thermo Scientific™ Orion™ ISE-Kalibrierstandards 0,1 M NH4Cl; 475 ml; CAS:(12125-02-9) Thermo Scientific™ Orion™ ISE-Kalibrierstandards | Fisher Scientific

Calculate the pH of a buffer prepared by mixing 300 cc of 0.3 M NH3 and 500 cc of 0.5 M NH4Cl . Kb for NH3 = 1.8 × 10^-5

SOLVED: What is the most efficient way to increase the pH and buffer capacity of an ammonium (NH4+) /ammonia (NH3) buffer? dilute the buffer add ammonium chloride add ammonia add NaOH(aq) add
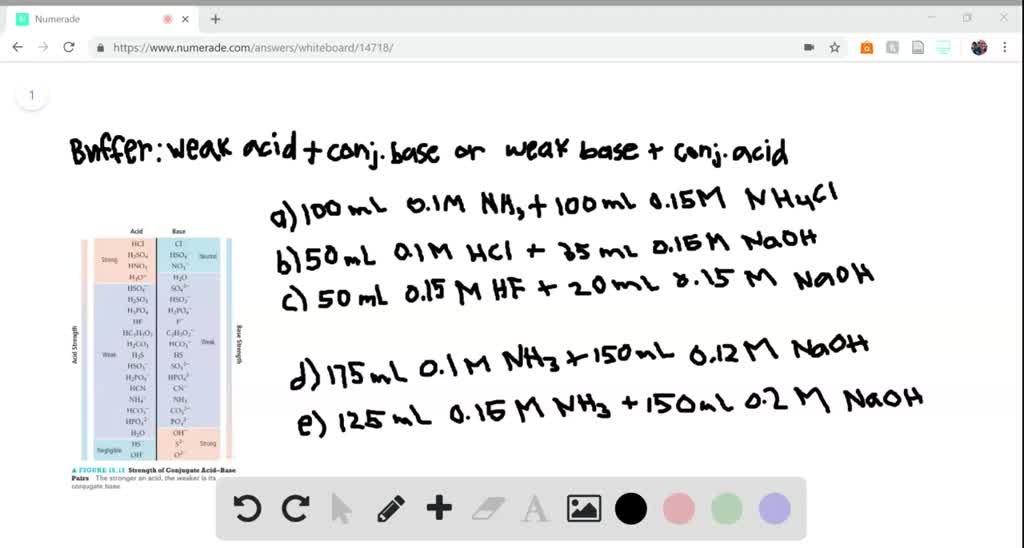
SOLVED:Determine whether the mixing of each pair of solutions results in a buffer. a. 100.0 mL of 0.10 M NH3; 100.0 mL of 0.15 M NH4Cl b. 50.0 mL of 0.10 M